We have a passion for unconventional solutions that bring your vision to life.
CDMO stands for Pharmaceutical Contract Customized R&D and Manufacturing Enterprise, which mainly provides pharmaceutical companies with services such as process R&D and preparation, process optimization, scale-up production, registration,verification production, and commercial production. The one-stop biopharmaceutical R&D and production platform of CDMO companies generally provides end-to-end services from target development to commercial production. At the same time, their quality management system needs to comply with the requirements of NMPA and the US FDA, which can greatly save time and cost for entrusting pharmaceutical companies.
Five basic requirements for CDMO
In order to successfully compete for production capacity, CDMO companies will first invest in their production and R&D workshops that meet GMP requirements. In terms of the decoration content of the widely needed GMP filling production workshop, it must first meet GMP requirements, but also meet process requirements, as well as construction and safety and health requirements. In the design of clean areas, five basic requirements should be paid attention to, namely:
Basic requirements for process layout
Basic requirements for cleanliness
Basic requirements for person of cleanrooms
Basic requirements for goods of cleanrooms
Basic requirements for purification channels and design
If it is a workshop that uses an old factory building for renovation, we must pay attention to the layout issues such as floor height and process piping.
 In operation areas with a higher degree of risk, such as filling, potting, sub-packaging, plugging, and capping in the filling workshop; the environment for process operations such as crushing, screening, mixing, and sub-packaging of sterile raw materials. Wiskind generally carries out interior design in accordance with the Class A clean area standards required by GMP.
In operation areas with a higher degree of risk, such as filling, potting, sub-packaging, plugging, and capping in the filling workshop; the environment for process operations such as crushing, screening, mixing, and sub-packaging of sterile raw materials. Wiskind generally carries out interior design in accordance with the Class A clean area standards required by GMP.
At the same time, Class B design is carried out in the background area where the Class A clean area for high-risk operations such as aseptic preparation and filling is located. Other less important operating links, such as: preparation of medicinal liquids or products that can be sterilized and filtered before filling, filtration of products, packaging materials that directly contact medicines, final cleaning, assembly or packaging of equipment, and sterilization processes The clean area where it is located is designed according to Class C and Class D.
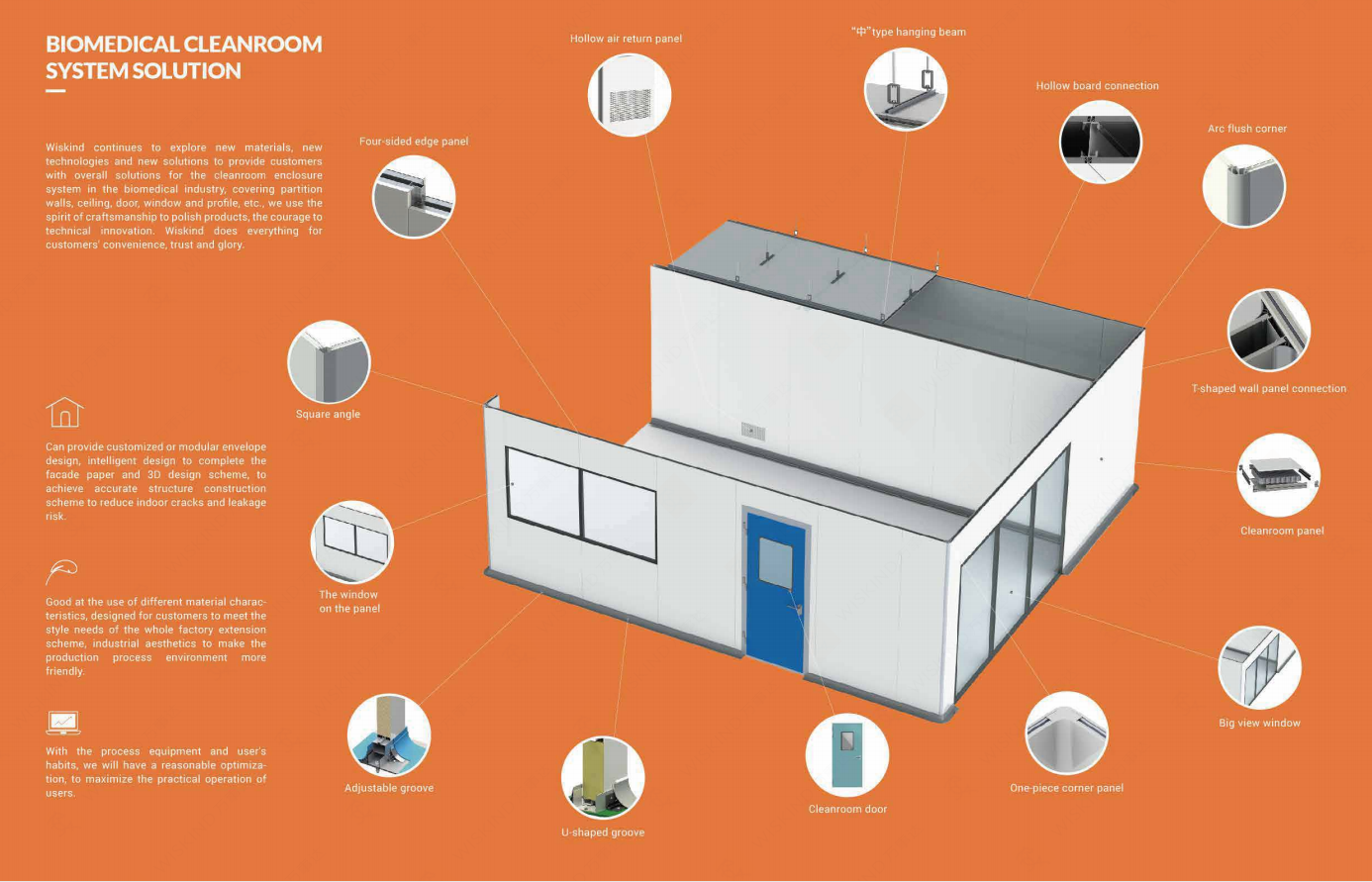
Wiskind has successfully provided comprehensive cleanroom enclosure system solutions for interenational biopharmaceutical CDMO companies, providing stable sterility guarantee for modern production and empowering partners in the biopharmaceutical industry.

Wiskind Cleanroom specializes in cleanroom enclosure system , ceiling system, cleanroom doors and windows and related product development, manufacturing, sales, consulting and services.